INTRODUCTION
The GHSG-HD21 trial in adult patients with newly diagnosed advanced-stage classical Hodgkin lymphoma (AS-cHL) compares BrECADD (Brentuximab vedotin, etoposide, cyclophosphamide, doxorubicin, dacarbazine, dexamethasone) to eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone). Superior treatment-related morbidity (TRMB) and at least non-inferior efficacy of BrECADD with high progression-free survival (PFS) rates after three years were presented previously. Fertility following chemotherapy is a major concern for these mostly young AS-HL patients. Therefore, we analyzed pregnancies and childbirth rates reported in HD21.
METHODS
This international open-label phase III trial included adult patients aged ≤ 60 yrs with AS-cHL. Patients were randomized in a 1:1 ratio to PET2-guided 4-6 cycles of either eBEACOPP or BrECADD. PET2 and PFS events were assessed by blinded panel review. Frequency of pregnancies among patients or their partners were analyzed in all female patients below 40 years and male patients below 50 years included in the ITT cohort for the safety endpoint TRMB. Descriptive statistics were used to report pregnancy rates. Childbirth rates per year were compared to German population data for women between 18-40 years in the years 2016-2020, obtained from the German Federal Statistical Office (Destatis). The trial was registered at clinicaltrials.gov (NCT02661503) and conducted according to ICH-GCP guidelines.
RESULTS
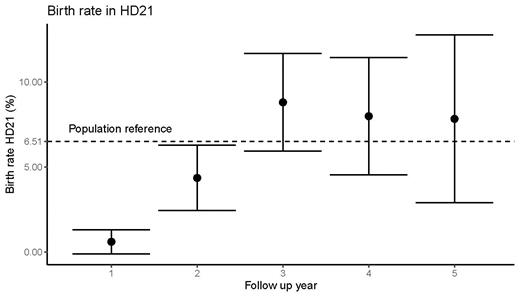
Between July 2016 and August 2020, we enrolled 1,500 patients from 9 countries and 233 trial sites. The final cohort for this analysis comprised 1200 patients (496 women and 704 men; 80.1% of ITT). Baseline characteristics were well balanced between treatment arms and median follow-up for this analysis was 40 months. 55.6% of the patients had cryocpreservation prior to chemotherapy (46.1% women, 76.4% men). At time of this analysis, there were 126 reported pregnancies during follow-up. Data was incomplete in 25 cases, and these were excluded from analysis. Overall, 97 (9.1%) patients had at least one documented pregnancy; 38 (7.2%) and 53 (9.8%) in eBEACOPP and BrECADD arm, respectively. Nine patients had more than one pregnancy. Compared to eBEACOPP, pregnancy rates following BrECADD were higher in males (5.7% vs. 2.5%) and females (13.2% vs. 11.8%). 8.2% of patients with reported pregnancy made use of cryopreservation. 82.2% of all pregnancies resulted in childbirth and 17.9% ended early (4% due to induced abortion; 13.9% due to abortion). Among patients with reported childbirth, there was a greater proportion of female patients vs. partners of male patients (63.9% vs. 36.1%); otherwise there were no notable differences between baseline characteristics or total cycles of chemotherapy in patients with reported pregnancy compared to the rest of the cohort. Median time from last day of chemotherapy until date of birth was 32 months (range 4-57). In women between 18 and 40 years, the childbirth rate per year increased during the first two years after treatment (first year: 0.6% [CI95: -0.1-1.3]; second year: 4.4% [CI95: 2.4-6.3]) and were numerically above the population reference (6.51%) starting from the third year (third year: 8.8% [CI95: 5.9-11.7]; fourth year: 8.0% [CI95: 4.5-11.4]; fifth year: 7.8% [CI95: 2.9-12.8]).
CONCLUSION
We report high rates of pregnancy following chemotherapy in the HD21 trial; approximately one tenth of patients below age 40 (female) or 50 (male) in the HD21 trial have reported a pregnancy during follow-up. Compared to eBEACOPP, pregnancy rates were twofold higher in partners of men who received BrECADD and slightly higher in women. Notably, childbirth rates in women after the second year of follow-up were comparable to the German population. Together with unparalleled primary cure rates achieved with BrECADD, our data supports its use in young patients with a desire to have children.
Disclosures
Ferdinandus:Roche: Honoraria. Greil:Roche: Honoraria, Research Funding. Molin:Takeda: Honoraria; BMS: Honoraria; Roche: Honoraria; MSD: Honoraria. Kerkhoff:AbbVie, Amgen, Zeneca: Honoraria; Takeda: Honoraria; Roche,Sobi: Honoraria; BeiGene, BMS, pharma: Honoraria. Topp:AbbVie: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Other: Travel support; Takeda: Research Funding; GenMab: Consultancy; Roche: Consultancy, Research Funding; Regeneron Pharmaceuticals, Inc.: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Other: Travel support, Research Funding. Vucinic:MSD: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sobi: Honoraria, Other: Travel/Accommodations/Expenses; Amgen: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Gilead/Kite: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Novartis: Consultancy, Honoraria; Abbvie: Honoraria. Schroers:GSK: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Borchmann:Bristol-Myers Squibb: Consultancy; Merck Sharp & Dohme: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Takeda Oncology: Consultancy, Research Funding; MPI: Research Funding.